Still #1: Lung Cancer Mortality
November is Lung Cancer Awareness Month. As of 2022, lung cancer remains the number one cause of cancer death globally (19%), making it the deadliest form of the disease. However, there is hopeful news. Large-scale randomized controlled trials, including the 2011 National Lung Screening Trial (NLST) and the 2020 NELSON study, have proven that Low-Dose CT (LDCT) screening can reduce lung cancer mortality by up to 20-30%. Furthermore, Coreline Soft's AI software supports these screening programs to operate more efficiently and consistently.
To date, LDCT-based lung cancer screening is the only scientifically proven method to reduce lung cancer mortality. This is not just a possibility; it is medical evidence established through long-term follow-up studies involving tens of thousands of subjects.
The Barrier to Early Detection: Radiologist Overload
Despite the proven efficacy of LDCT screening, its implementation in real-world clinical practice faces several barriers. The detection and analysis of pulmonary nodules are time-consuming tasks that demand intense concentration, thereby increasing the workload of radiologists.
A more critical issue is the consistency of readings. Inter-reader and inter-site variability is a key factor that undermines the reliability of screening programs. In national-level programs especially, where hundreds of medical institutions participate, differing reading standards and quality at each site can lead to uneven screening effectiveness.
This is not merely a technical problem; it is a systemic challenge directly linked to the cost-effectiveness of the screening program. Standardization and quality management are required across the entire screening process, from patient selection and image quality control to reading quality assurance and follow-up management.
AI Screening: Already a Global Standard
Against this backdrop, major countries worldwide are adopting AI technology as a core infrastructure for their National Lung Cancer Screening Programs. This is not simply about adding an automation tool; it is an approach that reconfigures the entire paradigm of the screening system.
[Korea] KNLCS: 40% Reduction in Inter-institutional Variability
The Korean Lung Cancer Screening (KLUCAS) pilot project is a large-scale program involving 70 hospitals and 200,000 people annually. Operating since 2017, the core of this program is the Quality Assurance (QA) Program led by the National Cancer Center (NCC).
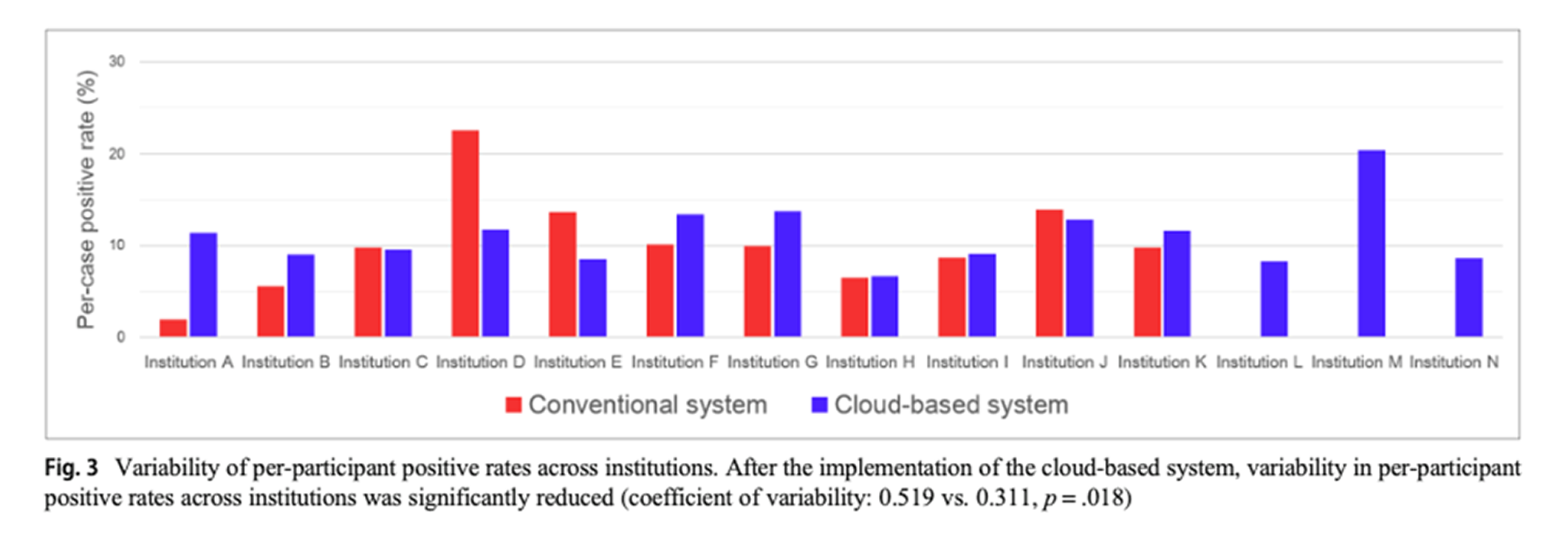
According to an NCC study comparing positive rates before and after the introduction of the AVIEW CLOUD system, inter-institutional variability decreased by
40% (0.519 → 0.311). This is significant clinical evidence showing that AI can go beyond assisting individual readers to standardize and homogenize the quality of an entire screening network.
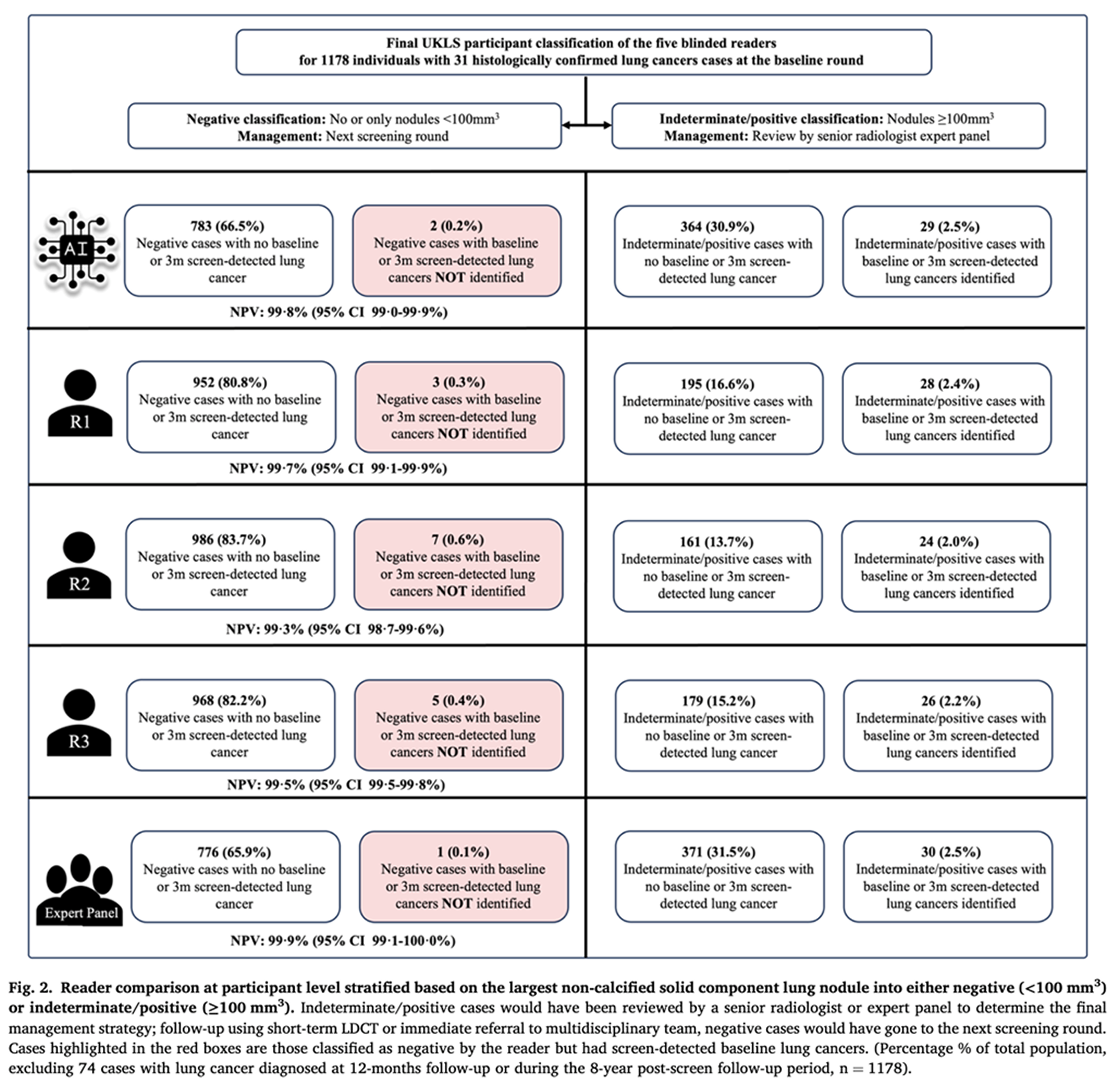
[UK] UKLS: 99.8% Sensitivity and 79% Workload Reduction
A validation study of AVIEW AI using 1,252 baseline LDCT data from the UK Lung Cancer Screening Trial (UKLS) was published in the
European Journal of Cancer in 2025.
The results showed a
99.8% sensitivity (missing only 1 of 31 cancers), and the AI's error rate (1) was lower than that of expert radiologists (3-7). This signifies that AI has already reached a clinically reliable level. Researchers analyzed that adopting an AI first-read model could lead to a
workload reduction of up to 79%. This enables a workflow redesign where AI filters out negative cases, allowing radiologists to focus on positive or suspicious cases.
[USA] FDA Approval: 70% Reduction in Reading Time
An independent reader study conducted during the US FDA 510(k) approval process (February 2023) directly compared results from 10 readers reading 'without AI' versus 'with AI'. The results showed a 34% increase in nodule detection and a 70% reduction in reading time. This demonstrates that AI does not replace radiologists but rather augments their capabilities.
[Europe] EU 4-IN-THE-LUNG-RUN: Establishing a Multinational Standard
The
EU 4-IN-THE-LUNG-RUN project(2022-2025) is a multinational screening program involving 8 hospitals in 6 countries and 26,000 subjects. It is an endeavor to establish a unified screening standard across Europe, and AVIEW LCS has been adopted as the core AI platform for this program.
[Germany] HANSE: Proving Accuracy in a Mobile CT Environment
The German HANSE project (2021-2024) is notable for its implementation in a Mobile CT and hybrid cloud (Edge Computing) environment. The HANSE study compared two AI tools, and research published in
Nature Scientific Reports in 2024 showed that AVIEW demonstrated more accurate quantitative results across all areas: nodule detection, volumetry, and classification.
[Academia] ESTI: Official Recognition by the European Society of Thoracic Imaging
The adoption of AVIEW LCS as the official online training program by the European Society of Thoracic Imaging (ESTI) is symbolic. It signifies that it has been recognized as a standard tool for educating European radiologists.
[Australia] AU-NLCSP: Implementing a 'Centralized Teleradiology' Model
Australia's NLCSP is a large-scale program with over 50 radiology practices and 330+ clinics. Australia's landmass is 35 times that of the Korean peninsula, and 30% of its population resides in rural and remote areas. In a Coreline x
LungScreen webinar last October 2025, Professor Siavash Es'haghi (LungScreen) emphasized that the core of this system is its centralized model and continuous QA loop. AVIEW LCS serves as the key AI engine maintaining quality and consistency within this system.
The Importance of AI Validation
Coreline's AVIEW has been cited or validated in 85 papers in the field of lung cancer screening and 269 papers in the field of "Big-3" screening (Lung Cancer/CAC/COPD). This demonstrates that AVIEW is one of the most actively researched and validated AI solutions worldwide.
AI performance can vary significantly based on training data, algorithm design, and clinical environment. Especially at a national screening level, AI must demonstrate consistent performance across diverse ethnicities, various CT scanners, and different scan protocols. The fact that AVIEW has been validated in diverse populations and medical environments—such as Korea, Europe, and Australia—signifies that it possesses this crucial generalizability.
Beyond Lung Cancer Screening to "Big-3" Integrated Screening
The field of lung cancer screening is currently in a paradigm shift from single-disease screening to comprehensive thoracic screening. The idea of evaluating the three major smoking-related diseases with a single LDCT scan was first proposed by the American Thoracic Society (ATS) in 2008 and is now globally accepted.
The "Big-3" Diseases: Lung Cancer, Cardiovascular Disease, Emphysema Lung Cancer, Cardiovascular Disease, and Emphysema are all closely related to smoking and can be assessed simultaneously with LDCT:
- Lung Cancer: Nodule detection and follow-up
- Cardiovascular Disease: Quantitative analysis of Coronary Artery Calcium (CAC)
- Emphysema: Quantitative analysis of Low Attenuation Area (LAA)
AVIEW LCS Plus is the world's first "Big-3" screening solution validated in clinical research.
Innovation in Screening Efficiency
This integrated approach dramatically improves cost-effectiveness. From the patient's perspective, they can be evaluated for multiple diseases in one visit. From the healthcare system's perspective, it increases participation rates while maximizing the reduction in all-cause mortality.
Cardiovascular disease is the #1 cause of death worldwide, and COPD is the 4th. The high-risk group for lung cancer is often the same high-risk group for these diseases. Therefore, "Big-3" integrated screening can be the most efficient screening strategy for this high-risk population. The Netherlands, through the EU's 4-IN-THE-LUNG-RUN program, is operating the B3 screening model at a national level, setting a benchmark for many other countries.
AI Realizes 'Quality' and 'Equity'
As we mark Lung Cancer Awareness Month in November, we once again emphasize the importance of early detection. LDCT screening is the only proven method to reduce lung cancer mortality, and its effectiveness is no longer in question.
The problem is no longer "Does it work?" but "How do we implement it effectively?" As major countries worldwide introduce national lung cancer screening programs, AI technology has become the core infrastructure for improving screening quality.
Quality and Equity
As the Australian model demonstrates, a well-designed, AI-integrated system can achieve both Quality and Equity simultaneously. It becomes possible for every patient to receive the same standard of screening quality, whether at a large urban hospital or a small rural clinic.
A Realistic Approach to Workforce Shortages
The shortage of radiologists is a worsening problem globally. As lung cancer screening programs expand, the demand for readings is surging. AI presents a realistic and validated approach to this problem. Through workflow innovations like AI first-read models, filtering of negative cases, and prioritization of suspicious cases, a limited workforce can screen more patients at a higher quality.
Data-Driven Continuous Improvement
Another advantage of an AI-integrated centralized system is data accumulation and continuous quality improvement. All screening data is accumulated centrally, and the entire program is continuously improved through real-time monitoring and feedback loops. In the case of Australia's LungScreen, over 6,000 scan datasets have been accumulated since the program began, which are used to analyze quality metrics such as radiation dose variability, incidental findings tracking, and recall success rates.
Coreline, Partner for National Screening Standards
Coreline Soft combines the only multi-disease, AI-based thoracic diagnostic platform with unmatched operational implementation experience. Deployed at leading healthcare providers worldwide, Coreline Soft's lungs+heart portfolio is FDA-cleared and has been chosen by seven national lung cancer screening programs for up to nine years.
The technology enables radiologists to achieve 70% faster read times while maintaining 97% clinical diagnostic sensitivity, with efficiency validated by over 290 peer-reviewed publications. The platform offers standardized Lung-RADS reporting, CAC scoring, LAA% quantification, longitudinal tracking, and seamless integration with all major PACS systems.



 List
List



